Os eletrodos das baterias de íons de lítio (LIBs) são compostos principalmente por materiais eletroquimicamente ativos, aditivos condutores, ligantes, coletores de corrente e outros componentes. Entre estes, os ligantes são um componente crítico dos eletrodos das LIBs. Os ligantes podem aderir firmemente os materiais ativos e condutores ao coletor de corrente, formando uma estrutura completa do eletrodo. Eles evitam o desprendimento ou a esfoliação dos materiais ativos durante os processos de carga e descarga, ao mesmo tempo que dispersam uniformemente os materiais ativos e os agentes condutores. Isso permite a formação de uma rede favorável de transporte de elétrons e íons, facilitando assim o transporte eficiente de elétrons e íons de lítio.
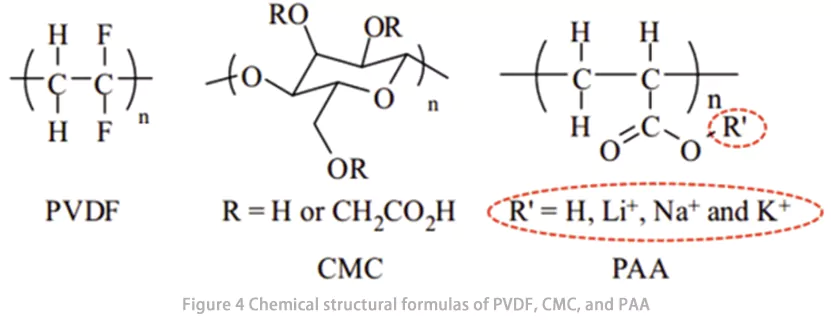
Atualmente, as substâncias utilizadas como ligantes de eletrodos incluem poli(fluoreto de vinilideno) ( PVDF) , carboximetilcelulose (CMC), borracha de estireno-butadieno (SBR), poli(vinilpirrolidona) (PVP), poli(metacrilato de metila) (PMMA), poli(acrilonitrila) (PAN), poli(ácido acrílico) ( PAA ), poli(álcool vinílico) (PVA), alginato de sódio (Alg), polímero de β-ciclodextrina (β-CDp), emulsão de polipropileno (LA132), poli(tetrafluoroetileno) ( PTFE ), e assim por diante, bem como derivados funcionalizados dos polímeros ou copolímeros acima mencionados, formados por monômeros.
Em eletrodos de bateria de íons de lítio (LIB), o desempenho ideal do ligante deve incluir:
(1) estabilidade química e eletroquímica em um determinado sistema eletrodo/eletrólito, resistência à corrosão do eletrólito e nenhuma ocorrência de reações redox dentro da faixa de tensão operacional;
(2) Deve apresentar boa solubilidade, com rápida taxa de dissolução e alta solubilidade em solventes, e os solventes necessários devem ser seguros, ecologicamente corretos e não tóxicos, sendo preferidos os solventes à base de água;
(3) Deve ter viscosidade moderada para facilitar a mistura da pasta e manter a estabilidade da pasta, além de possuir forte adesão, resultando em eletrodos com alta resistência ao descascamento, excelentes propriedades mecânicas e baixo uso de ligante;
(4) Deve demonstrar boa flexibilidade para tolerar a flexão durante o manuseio do eletrodo e as mudanças de volume das partículas do material ativo durante os ciclos de carga-descarga dos LIBs;
(5) Deve ser capaz de formar uma rede condutora ideal com agentes condutores, levando a eletrodos com boa condutividade elétrica e capacidade de condução de íons de lítio;
(6) Deve estar amplamente disponível e ter baixo custo.
Este artigo resume as conquistas recentes de pesquisas relacionadas aos ligantes de eletrodos LIB, com foco na introdução dos mecanismos de adesão dos ligantes em eletrodos e dos ligantes à base de óleo e água comumente usados nos eletrodos LIB atuais.
1 Mecanismo de adesão de ligantes em eletrodos de bateria de íons de lítio
O processo de produção de eletrodos LIB normalmente envolve quatro etapas: misturar vários materiais (incluindo materiais ativos de eletrodo) em um solvente para formar uma pasta de bateria, revestir a pasta em um coletor de corrente, secar e rolar. Acredita-se que os eletrodos LIB sejam compostos por três componentes: partículas de material ativo (MA) que servem como fontes de íons e elétrons, espaço poroso preenchido com eletrólito para condução iônica e domínios ligantes de carbono (CBD) que fornecem condutividade.
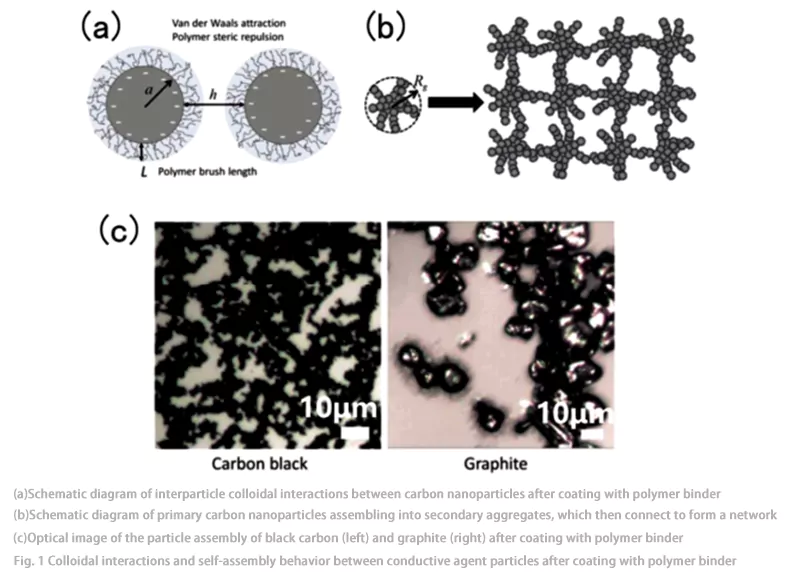
O CBD é tipicamente composto por nanopartículas de carbono conectadas por um ligante polimérico (Fig. 1), enquanto a pasta precursora necessária para a preparação do eletrodo consiste em partículas de material ativo (MA) de tamanho micrométrico suspensas dentro do CBD. O CBD influencia diretamente a eficiência do transporte de íons e elétrons no eletrodo, bem como a qualidade das camadas de passivação (por exemplo, filmes de interfase eletrolítica sólida [SEI] e interfase eletrolítica catódica [CEI]) formadas na superfície dos materiais ativos em contato com o eletrólito. Portanto, o CBD desempenha um papel crítico no processo de fabricação do eletrodo: CBD insuficiente leva à conectividade deficiente do eletrodo, resultando em transporte inadequado de elétrons e resistência mecânica insuficiente do eletrodo; CBD excessivo aumenta o peso próprio e o volume da bateria, podendo até mesmo retardar o transporte de íons.
Zielke et al. empregaram uma nova abordagem combinando tomografia computadorizada de raios X (TC) e design virtual para comparar as influências de dois modelos de domínio ligante de carbono (CBD) na área de superfície, tortuosidade e condutividade elétrica do filme de interfase eletrolítica sólida (SEI) em eletrodos em baterias de íons de lítio (LIBs) durante os estados de carga e descarga. Os resultados demonstraram que o teor de CBD impacta significativamente os parâmetros de transporte das LIBs tanto em condições de carga quanto de descarga, enquanto a morfologia do CBD exerce um efeito crítico apenas no estado de descarga.
O grupo de Prasher propôs um modelo microrreológico que incorpora interações coloidais interpartículas e interações hidrodinâmicas, o qual foi utilizado para prever a viscosidade de suspensões de nanopartículas de carbono condutoras e ligantes poliméricos, bem como de toda a pasta anódica. Os resultados revelaram que as interações entre as partículas de nanopartículas de carbono dependem amplamente da proporção entre partículas e ligante polimérico e do peso molecular do ligante polimérico. Além disso, as mudanças nas interações interpartículas refletem-se claramente na estrutura automontada das partículas, que por sua vez se manifesta na viscosidade da pasta.
Srivastava et al. elucidaram a influência da adesão entre o material ativo (AM) e o domínio de ligação de carbono (CBD) e a coesão dentro do CBD na microestrutura do eletrodo e propriedades-chave relacionadas ao transporte eletroquímico (como tortuosidade do transporte iônico, condutividade eletrônica e área interfacial disponível entre o material ativo (AM) e o eletrólito) por meio de simulações de dinâmica de partículas e dinâmica de coloides.
2 ligantes de eletrodos comuns para baterias de íons de lítio
2.1 Poli(fluoreto de vinilideno) (à base de óleo)
O poli(fluoreto de vinilideno) (PVDF) é um dos primeiros ligantes utilizados. Apresenta alta resistência mecânica e ampla janela de estabilidade eletroquímica, tornando-o amplamente utilizado como ligante para eletrodos de baterias em diversos sistemas, incluindo baterias de íons de lítio (LIBs). Na produção em larga escala da indústria de baterias de íons de lítio, compostos orgânicos fortemente polares, como N-metil-2-pirrolidona (NMP) e N,N-dimetilformamida (DMF), são comumente utilizados como solventes. O PVDF é primeiramente dissolvido nesses solventes para formar uma solução solúvel em óleo, que é então utilizada como ligante para baterias de lítio.
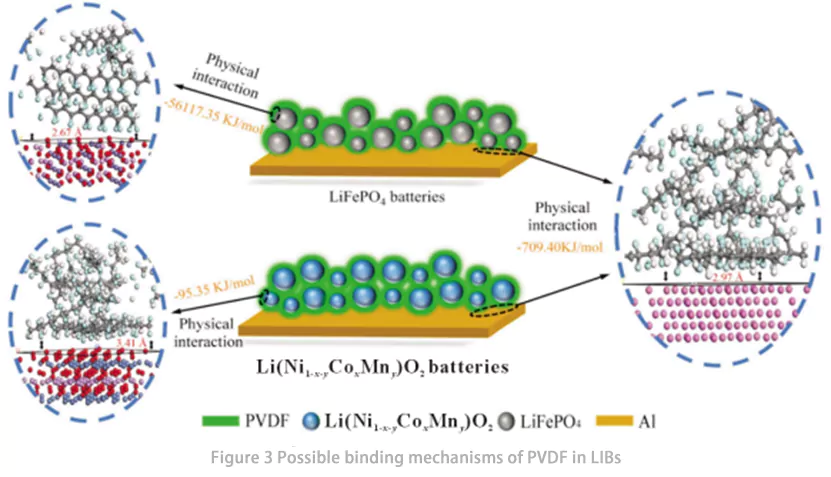
Zhong et al. investigaram o mecanismo de ligação entre o material ativo (AM) e o poli(fluoreto de vinilideno) (PVDF) em baterias de íons de lítio (LIBs) por meio de simulações da teoria do funcional da densidade (DFT) e análise da interface de ligação entre as partículas de AM e o ligante em eletrodos de LIB (Fig. 2). Os resultados das simulações de processo e cálculos teóricos indicaram que, em baterias de LiFePO₄ (LFP), a interação de ligação entre LFP e PVDF foi significativamente mais forte do que entre PVDF e alumínio (Al), enquanto em baterias de Ni-Co-Mn (NCM), a interação de ligação entre NCM e PVDF foi mais fraca do que entre PVDF e Al. As análises de microscopia eletrônica de varredura (MEV) e espectroscopia eletrônica Auger (AES) revelaram que, em baterias LFP, o PVDF foi distribuído principalmente na superfície da LFP, indicando baixo desempenho adesivo do PVDF em baterias LFP. Em contraste, em baterias NCM, o PVDF foi distribuído uniformemente nas superfícies dos materiais ativos e do Al, indicando um bom desempenho adesivo do PVDF em baterias NCM. Esses resultados sugerem que o desenvolvimento de novos ligantes à base de PVDF para LIBs deve priorizar o aprimoramento da interação de ligação entre o ligante e o Al. Além disso, eles confirmaram que as interações entre AM, Al e PVDF em LIBs são principalmente físicas, e não químicas.
2.2 Carboximetilcelulose e borracha de estireno-butadieno (à base de água)
A carboximetilcelulose (CMC) é um polímero linear derivado da celulose, formado pela substituição diferencial da celulose natural por grupos carboximetila. Como um ácido fraco poliprótico, a CMC pode se dissociar para formar grupos funcionais aniônicos carboxilato. Além disso, a presença de grupos carboximetila torna a CMC mais solúvel em água em comparação com a etilcelulose (EC), metilcelulose (MC) e hidroxietilcelulose (HEC). Essa solubilidade em água permite que a CMC seja usada na produção de eletrodos à base de água, oferecendo vantagens sobre o PVDF em termos de baixo custo, não toxicidade e fabricação ecologicamente correta. Os grupos de ácido carboxílico livres na CMC podem interagir com grupos hidroxila nas superfícies de materiais como silício/carbono, facilitando a formação de uma rede ideal de domínio ligante de carbono (CBD) em eletrodos. Além disso, a CMC é caracterizada por baixo custo, boa estabilidade térmica e respeito ao meio ambiente, tornando-a um potencial ligante para ânodos em baterias de íons de lítio (LIBs).
Estudos de Lee et al. demonstraram que suspensões de grafite utilizando carboximetilcelulose (CMC) com menor grau de substituição como ligante apresentam melhor estabilidade de suspensão. Isso ocorre porque a CMC com menor grau de substituição possui maior hidrofobicidade, o que aumenta sua interação com a superfície da grafite no meio aquoso. Drofenik et al. demonstraram que um ânodo de grafite utilizando uma pequena quantidade de CMC (com fração mássica de 2%) pode obter o mesmo efeito que uma grande quantidade de ligante poli(fluoreto de vinilideno) (PVDF) (10%). Além disso, isso não afeta a intercalação/desintercalação normal de íons de lítio no eletrodo de grafite ou a formação do filme de interfase eletrolítica sólida (SEI). Essas descobertas indicam que o uso de CMC reduz a quantidade necessária de ligante, o que é benéfico para melhorar a densidade energética dos eletrodos de bateria de íons de lítio (LIB), tornando a CMC um excelente ligante anódico para LIBs.
No entanto, o ligante aquoso de carboximetilcelulose (CMC) apresenta forte rigidez e fragilidade. Após a secagem a vácuo, rachaduras são visivelmente presentes na superfície dos eletrodos que utilizam CMC como ligante, o que pode até levar a lacunas entre o revestimento do material ativo e o coletor de corrente, causando "desprendimento de material" no eletrodo. Para abordar essa questão, Liu et al. utilizaram borracha de estireno-butadieno (SBR) como aditivo flexível para o ligante CMC. Eles compararam os efeitos dos ligantes compósitos SBR-CMC com ligantes PVDF tradicionais na estabilidade cíclica de ânodos de silício (Si) e investigaram as propriedades mecânicas e o comportamento de intumescimento dos ligantes compósitos SBR-CMC em soluções eletrolíticas. Os resultados mostraram que a adição de SBR reduziu efetivamente a fragilidade do eletrodo. Comparados aos ligantes PVDF, os ânodos de Si que utilizam ligantes compósitos SBR-CMC exibiram um módulo de Young menor, maior alongamento máximo e maior força de adesão ao coletor de corrente.
Pesquisas do grupo de Dahn demonstraram que eletrodos de silício (Si) fabricados com ligantes compostos de SBR-CMC apresentam melhor retenção de capacidade em comparação com aqueles fabricados utilizando apenas ligantes de CMC. O estudo revelou que, como o CMC é um polímero altamente rígido e quebradiço, o ligante aquoso de CMC apresenta bom desempenho como ligante em eletrodos com alta taxa de variação de volume de partículas de material ativo. No entanto, o ligante aquoso de CMC absorve menos eletrólitos de carbonato orgânico do que o PVDF, o que pode afetar a capacidade de taxa de eletrodos que utilizam CMC como ligante.
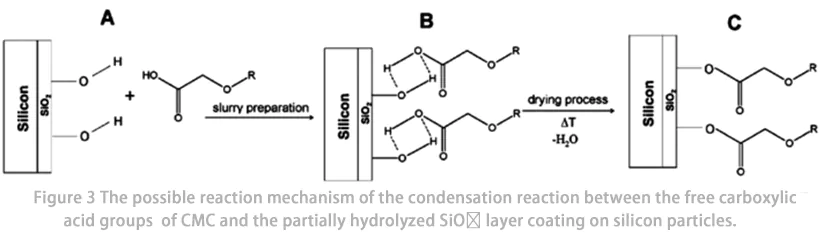
Além disso, a CMC também é utilizada como aditivo para melhorar a estabilidade do ciclo de ânodos de baterias de íons de lítio (LIB) (por exemplo, ligas de Si e Sn), que apresentam variações significativas de volume durante o ciclo da bateria. O mecanismo para a melhoria do desempenho do ciclo é considerado como sendo: (1) a ligação entre partículas de Si e partículas aditivas condutoras carbonáceas por meio de cadeias de CMC; (2) a formação de ligações covalentes estáveis (Fig. 3) ou ligações de hidrogênio autorregenerativas na superfície das partículas de Si pela CMC.
2.3 Ligantes à base de ácido poliacrílico (à base de água)
O poli(ácido acrílico) (PAA) é um polímero solúvel em água formado pela polimerização de monômeros de ácido acrílico. Devido à presença de um grande número de grupos de ácido carboxílico em sua estrutura (Fig. 4), o PAA pode formar fortes interações com materiais ativos e folhas de alumínio, exibindo, assim, excelentes propriedades de ligação. Ele serve como um ligante promissor de alto desempenho para eletrodos de baterias de íons de lítio (LIB). Além disso, durante o ciclo das LIBs, o PAA facilita a formação de uma interface catódica-eletrólito (CEI) estável, o que aumenta a estabilidade do ciclo das LIBs.
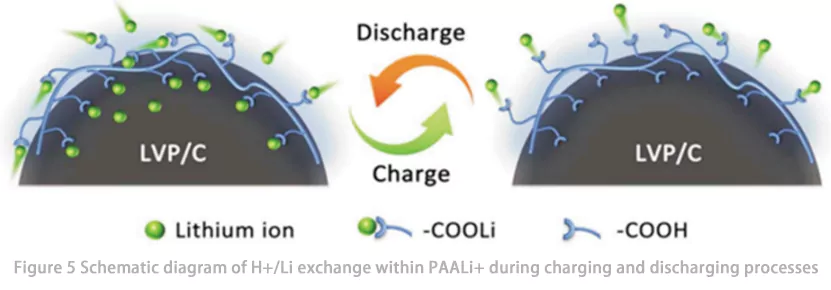
Su et al. utilizaram pela primeira vez poli(alil lítio) (PAALi) como um ligante para Li₃V₂(PO₄)₃ (LVP) e investigaram o comportamento de transporte de Li⁺ em PAALi e sua influência no desempenho eletroquímico de baterias de íons de lítio (LIBs). Os resultados mostraram que o novo ligante PAALi exibiu excelente estabilidade em eletrólitos orgânicos e boa adesão a todos os componentes do eletrodo, formando assim uma rede condutora contínua nos eletrodos. A bateria LVP usando o novo ligante PAALi manteve uma taxa de retenção de capacidade de 91% após 1400 ciclos a 10 °C. Por meio de testes e análises como espectroscopia de infravermelho com transformada de Fourier (FTIR) e espectroscopia de fotoelétrons de raios X (XPS), eles demonstraram que o ligante PAALi promove o transporte de Li⁺ na interface do eletrodo por meio de uma reação de troca reversível H⁺/Li⁺ entre os grupos –COOH e –COOLi (Fig. 5). Esse transporte altamente sinérgico de elétrons e Li⁺ na bateria PAALi-LVP melhora o desempenho cinético do eletrodo e fornece um rápido processo redox capacitivo, permitindo atingir uma excelente capacidade de taxa de 107 mAh/g a 70 C.
Chong et al. investigaram o desempenho eletroquímico de meias-células e células completas em sistemas de baterias de grafite/LiFePO₄ usando PAA com SBR adicionado como ligante versus PVDF como ligante. Os resultados mostraram que PAAX (onde X = H, Li, Na ou K) poderia efetivamente melhorar a eficiência coulômbica inicial, a capacidade reversível e a estabilidade do ciclo de baterias de grafite/LiFePO₄ em comparação com PVDF. A adição de uma pequena quantidade de SBR (0,5%–3,0%) impediu a formação de rachaduras frágeis na superfície do eletrodo após a secagem. Entre os ligantes da série PAAX, PAALi e PAANa exibiram melhor desempenho de bateria, o que foi atribuído à sua capacidade de formar conformações poliméricas mais favoráveis nos compósitos de eletrodo (relacionados ao CEI). Além disso, os ligantes da série PAAX à base de água podem reduzir o custo de fabricação de baterias de grafite/LiFePO₄ e minimizar os danos ambientais.
3 Resumo e Perspectivas
Embora os ligantes em eletrodos de baterias de íons de lítio (LIB) sejam materiais eletroquimicamente inativos, eles podem co-formar uma estrutura de domínio de carbono-ligante (CBD) com nanopartículas de carbono condutoras. Quando a adesão entre o ligante e o coletor de corrente é favorável, o ajuste da coesão do CBD e da adesão entre o material ativo (MA) e o CBD pode formar uma rede condutora de CBD de alta qualidade. Isso não apenas confere ao eletrodo forte resistência mecânica e resistência ao descascamento, mas também estabelece uma rede condutora dentro do eletrodo que facilita a condução de elétrons, aumentando assim a eficiência do transporte de elétrons. Além disso, ajuda a aumentar a área interfacial AM-eletrólito para condução iônica, reduz a tortuosidade do transporte iônico dentro do eletrodo e melhora a qualidade das camadas de passivação (por exemplo, filmes de interface eletrolítica sólida [SEI] e de interface eletrolítica catódica [CEI]) formadas na superfície do AM em contato com o eletrólito. Coletivamente, esses efeitos exercem uma influência significativa no desempenho eletroquímico do eletrodo.
Atualmente, os ligantes comumente usados para eletrodos LIB incluem principalmente ligantes à base de óleo, representados por PVDF, e ligantes à base de água, representados por CMC, SBR e PAA, conforme discutido neste artigo. O PVDF exibe boa adesão aos coletores de corrente, e seu peso molecular pode ser ajustado pela modificação do grau de polimerização do fluoreto de vinilideno (VDF), regulando assim suas propriedades de ligação. Atualmente, é amplamente utilizado na produção de eletrodos para diversos sistemas de baterias. Comparados ao ligante solúvel em óleo PVDF, ligantes à base de água, como CMC, SBR e PAA, não requerem o uso de solventes orgânicos durante a aplicação prática, evitando assim a poluição ambiental e danos à saúde dos operadores causados por vapores de solventes orgânicos em alta temperatura. Entre os ligantes à base de água, o CMC, como um derivado da celulose, é caracterizado por sua ampla disponibilidade e baixo custo, atendendo aos requisitos de LIBs de baixo custo. Também pode servir como um aditivo para melhorar a estabilidade do ciclo de ânodos de silício, mostrando amplas perspectivas de aplicação. O ligante PAALi apresenta bom desempenho de ligação e pode repor o consumo de lítio ativo durante o ciclo de LIBs, demonstrando um potencial de desenvolvimento significativo. Espera-se que ele abra um novo caminho para o desenvolvimento de ligantes de alto desempenho para LIBs.
Referências: Fu Tiantian, Tao Fuxing, Li Chaowei, Zhang Yang, Wang Jiuzhou. Progresso da Pesquisa em Ligantes para Baterias de Íons de Lítio[J]. Tecnologia de Energia, 2023, 47(5): 570-574.